OPEN
 ACCESS
ACCESS
Fisher Ethnotaxonomy for Elasmobranchs Captured Along the
Brazilian Amazon Coast
Keyton K. F.
Coelho1,2*, Getulio Rincon3,
Arkley M. Bandeira1, Márcio L. V.
Barbosa-Filho4, Natascha Wosnick5, Rafaela M. S. de Brito1,2, Ana R. O. P. Nunes1,2 and Jorge L. S. Nunes1,2
1Departamento
de Oceanografia e Limnologia, Universidade Federal do Maranhão, São Luís,
Brazil. 2Programa de Pós-Graduação em Biodiversidade e Biotecnologia
da Amazônia Legal - Rede BIONORTE, Universidade Federal do Maranhão, São Luís,
Brazil. 3Curso de Engenharia de Pesca, Universidade Federal do
Maranhão, Pinheiro, Brazil. 4VP Eco Engenharia & Meio Ambiente,
Taubaté, São Paulo, Brazil. 5Departamento de Zoologia, Universidade
Federal do Paraná, Curitiba, Brazil.
*keytonfc@yahoo.com.br
Received April 8, 2022 | Accepted
October 5, 2022 | Published February 11, 2023
Ethnobiology Letters 2022 13(1):79–99 | DOI
10.14237/ebl.13.1.2022.1819
Abstract The diversity of
popular names used in fish nomenclature off the Brazilian coast makes it
difficult to identify species, and many names have their origins in Indigenous
languages, mainly Tupi-Guarani. This study sought to understand and update the
list of the most popular names and assess some ethnotaxonomic patterns employed
by artisanal fishers from the Brazilian Amazon Coast in naming elasmobranchs.
Interviews with 314 fishermen from 17 coastal municipalities were carried out
employing a semi-structured form, banners, and photographic records of local
elasmobranch species, addressing characteristics applied to species
identification. A total of 130 ethnospecies were identified (113 names in
Portuguese and 17 of Tupi-Guarani origin) for the identification of 22 and 18
species of sharks and rays, respectively. The highest degree of homonyms occurs
interspecifically for the Dasyatidae, Mobulidae, Pristidae, Urotrygonidae,
Carcharhinidae, Sphyrnidae and Triakidae families. Sphyrna tiburo and Hypanus
guttatus comprised the taxa with the highest diversity of common names.
Morphological characteristics such as shape, colors, texture, and size of
certain body parts are the ethnotaxonomic patterns most applied in shark and
ray identification. We conclude that the use of common names for elasmofauna
facilitates communication between fishers and that the scientific approach to this local ecological knowledge is
fundamental for the management and sustainability of fisheries in the long
term.
Keywords Ethnobiology, Ethnospecies, Local Ecological
Knowledge, Chondrichthyes
Introduction
Traditional communities
inhabiting coastal Brazilian regions attribute a great diversity of popular
names to marine fish and other nature elements (Barbosa-Filho et al. 2021;
Freire and Carvalho-Filho, 2009). The diversity of names employed by fishers and
fish consumers is due to multiple factors, including country size, regional
disparities, colonization processes, and the complexities of Brazilian culture
(Amorim 2005; Freire and Pauly 2005; Mourão and Barbosa-Filho 2018; Rodrigues
2016). Many of the popular plant and animal names in Brazil have their origins
in Tupi-Guarani linguistics (Barbosa 1951). Since colonization, the Portuguese
language spoken in Brazil has been marked by the Tupi linguistic trunk, which
is manifested in the names of places, landscape landmarks, animals, plants, and
food (Dietrich and Noll 2016a). This stems from the relations established
during the colonial period between the Portuguese and Tupi-Guarani inhabiting
the Brazilian coast, especially the Tupinambá people, whose loans and cultural
exchanges were historically documented through linguistic contacts and the
absorption of numerous Amerindian words in the Portuguese language spoken in
Brazil (Dietrich 2016).
In the first half
of the 16th century, Tupinambá was widely spoken in Brazilian
coastal zones and estuarine areas, as well as in some inland areas (Rodrigues
2016). Its dispersion followed the migratory flows of Indigenous people and was
adopted in Jesuit missions between the 16th and 17th
centuries, while other languages of Tupi origin were spoken in other regions of
the country (Dietrich and Noll 2016b). However, Tupinambá fell into disuse with
the genocide of Tupinambádue to epidemics and the catechization process and
subsequent religious assimilation (Rodrigues 2016). From the 19th
century, the term "Tupi" refers to a complex linguistic combination,
comprising Tupinambá, on which most colonial languages are based on, the
Brasilic language used in Jesuit missions, the language spoken in São Paulo on
the Piratininga plateau—the first colonizing nucleus towards the Southeast, and
the Amazonian language used in settlements of Indigenous Peoples of different
ethnic origins in the Grão-Pará Jesuit missions (Dietrich 2016).
"Tupi" has also been applied as a generic term since the 16th
century to designate Indigenous populations along the Brazilian coast
(Rodrigues 2016). With the intensified contact between the Portuguese and
Brazilian Indigenous Peoples, both were learning to use each other's language,
mixing, exchanging, and building a common language that has influenced the
current language spoken in Brazil.
The growing need
to include traditional communities and natural resources users in biodiversity
management and conservation has shown traditional ecological knowledge to be a
promising tool (Barbosa-Filho et al. 2021; Ferreira-Araujo et al. 2021; Giareta
et al. 2021; Rodrigues et al. 2021; Silva et al. 2021). For example,
ethnotaxonomy can be applied to improve and adapt management plans, as the use
of inclusive language increases the chances of traditional communities
understanding what is being proposed and for which species. Ethnotaxonomy
translations can also fill knowledge gaps regarding target-species biology and
ecology, particularly in data-poor countries such as Brazil (Ladislau et al.
2021; Mourão and Barbosa-Filho 2018). Concerning artisanal fisheries,
traditional fisher knowledge is of great value, as fishing is spread out and
landings are difficult to monitor. In Brazil, elasmobranch fishing is a
traditional activity, with several coastal communities engaged in the capture
and trade of sharks and rays (Aragão et al. 2019; Barbosa-Filho et al. 2019; Barbosa-Filho
et al. 2021; Carvalho et al. 2018; Martins et al. 2018). Not entirely a
subsistence activity, elasmobranch fishing guarantees the financial gain of
many families under socio-economic vulnerability conditions, as well as food
security in many regions of the country (Araujo et al., 2020; Dias et al. 2016;
Martins et al. 2018; Nunes et al. 2005; Pinto et al. 2015; Viana and Souza
2019). The point of concern is that sharks and rays are now among the most
threatened vertebrates worldwide, with population declines that seriously
compromise their sustainable use (Dulvy et al. 2021; Pacoureau et al. 2021).
The situation is critical in Brazil, as official fisheries statistics are
absent since 2011, and legislation towards elasmobranch conservation is rarely
met, mainly due to a lack of enforcement and incentive programs aiming at
reducing elasmobranch catches. Moreover, the vast majority of species captured
incidentally are retained and traded, posing additional pressure to
elasmobranchs throughout the Brazilian Exclusive Economic Zone.
The Brazilian
Amazon coast (BAC) is listed as a global conservation hotspot, mainly due to
the significant number of local endemic species threatened with extinction
(Dulvy et al. 2014). The region has a large artisanal fleet that captures
elasmobranchs throughout the year, catching mostly juveniles and pregnant
females (Almeida et al. 2000; Araujo et al. 2020; Gonçalves 2004; Lessa et al.
1999; Lessa and Silva 1992; Nunes et al. 2016). Members of fishing communities
are mostly citizens suffering great social vulnerability, marginalization, and being
deprived of access to basic health, education, and adequate living conditions.
The management of endangered species in the Brazilian Amazon region is very
challenging, and human dimensions are constantly overlooked in decision-making
processes. In order to improve shark and ray management in the region,
traditional communities should be not only considered in decision-making
processes, but also their knowledge and demands in conservation planning. This
includes access to regional ethnotaxonomy, especially considering the barriers
imposed by poor access to basic education and the complexity of the language
used in legal/punitive measures (e.g., list of banned species).
In this context,
the present study aims to update the list of popular names of sharks and rays
used by traditional communities inserted in the BAC and identify ethnotaxonomic
patterns applied in the identification and classification of captured species marketed
by local artisanal fleets as a way to reduce the linguistic distance between
academia, policy makers and fisheries resource users.
Material and Methods
Study Area
The data were collected along
the coast of the state of Maranhão, which extends from the mouth of the Gurupi
River to the mouth of the Parnaíba River, approximately 640 km in length
(IMESC, 2020). This coastline comprises three Environmental Protection Areas
(EPA) with 35 municipalities, an estimated population of over two million
inhabitants, and is part of the BAC (IBGE, 2020; Figure 1).
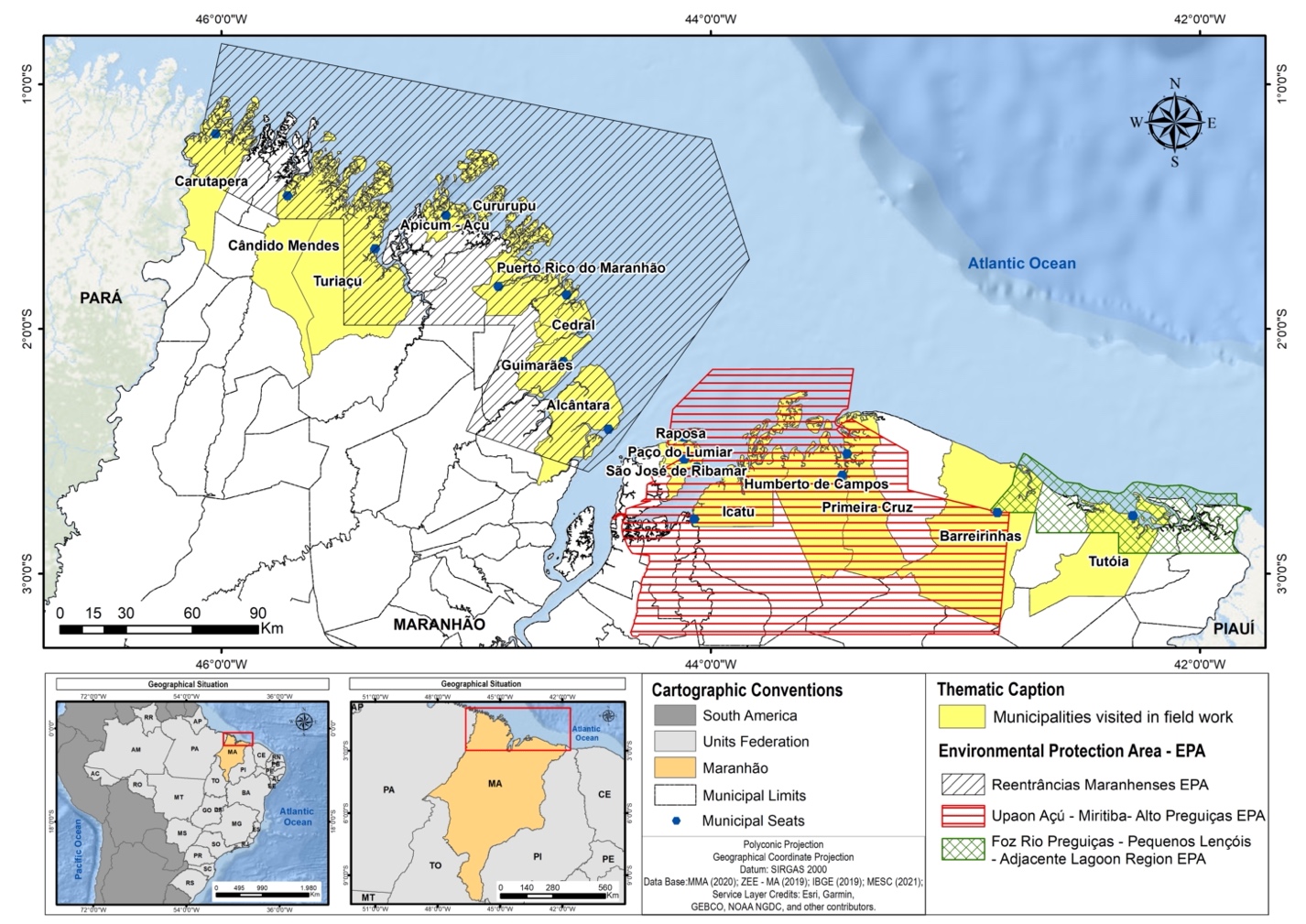
Figure 1 Delimitation
of Environmental Protection Areas and the 17 municipalities that make up the
study area on the coast of the state of Maranhão, located on the Brazilian
Amazon Coast. Credits: Brenda S. S. Nunes, 2021.
The western coast
comprises the Reentrâncias Maranhenses Environmental Protection Area (EPA).
This area is characterized by an expressive set of islands, peninsulas, and
bays, cut by rivers, streams and tidal channels filled with clay and silt that
favor mangrove development (Castro et al. 2019) with high fishing resource
productivity, representing a source of food and work for most coastal and
riverside populations, especially low-income communities (IMESC 2020). The
central part comprises the Golfão Maranhense, an estuarine complex formed by
three bays, several river discharge sites and the island of Maranhão (Castro et
al. 2019), as well as the Upaon Açu-Miritiba-Alto do Rio Preguiças EPA. The
latter displays paramount importance concerning the region's high commercial
value fishing resources, such as Acoupa weakfish Cynoscion
acoupa (Lacepède, 1801) and Serra Spanish mackerel Scomberomorus
brasiliensis Collette, Russo & Zavala-Camin, 1978 (IMESC 2020). The
eastern coast comprises the Foz do Rio das Preguiças - Pequenos Lençóis -
Adjacent Lagoon Region EPA (IMESC 2020), marked by a straight coastline, tidal
terraces, fixed and mobile dunes, mangroves, beaches, bays, islands, coves, and
the Parnaíba River delta (El-Robrini et al. 2018; Figure 1).
Data collection and analysis
Monthly interviews were
carried out with artisanal fishers from December 2019 to October 2020 in the
main Alcântara, Apicum Açú, Barreirinhas, Cândido Mendes, Carutapera, Cedral,
Cururupu, Guimarães, Humberto de Campos, Icatú, Paço do Lumiar, Porto Rico,
Primeira Cruz, Raposa, São José de Ribamar, Turiaçú and Tutóia ports (Figure
1). The interviews took place over three days with a daily effort of eight
hours at each location, when the interviewees were performing fishing gear
maintenance, vessel repairs or following fish landings.
The interviews
took place individually through a semi-structured form, visually stimulated by
banners (Figure 2) and photographic records of local elasmobranchs (see Wosnick
et al. 2019), focusing on their common names and external characteristics used
for species identification. During the interviews, fishers were also asked
about the species that were caught in abundance in the past and that have
disappeared, species currently hardly caught at all and species not recorded
for the region. The obtained information was compared with available literature
(Almeida 2008; Almeida et al. 2011; Martins-Jura et al. 1987; Marceniuk et al.
2020; Nunes et al. 2005; Nunes et al. 2011; Stride et al. 1992). In addition,
an additional search on local fauna records from the 17th century
was carried out to understand the origins, historical records and diversity of
popular names applied to elasmobranchs.

Figure 2 Interviews
and data collection in the municipalities of Carutapera (A) - West Coast,
Tutóia (B) - East Coast and Raposa (C) - Golfão Maranhense, in the state of
Maranhão. Credit: Keyton K. F. Coelho, 2020.
Data were quantitatively
analyzed to obtain the total common names and relative frequencies (Fr) of
citations for each species, as well as the total percentage of each common name
cited in relation to all species identified by fishers.
Linguistic considerations
In the present study, common
names were considered non-scientific nomenclature employed by fishing
communities and fish consumers for the identification of morphological entities
and, therefore of no official taxonomic nature. Synonymy was considered as the
use of different common names applied to the same species (Minelli 1999), while
homonymy was considered when at least two distinct species were associated with
the same common name (Papavero 1994). Polysemy cases were associated with
generalized naming conditions regarding initial species identification (e.g.,
“arraia” or “cação;” Martins 2015). The richness of common names was evaluated
by the sum of synonyms and homonyms (Minelli 1999). The observed variation was
subtle in many cases, but details were also considered as a possible variation
of diachronic origin, which consists of slightly modified forms due to
divergences over time (e.g., “arraia-lixa” or “raia-lixa”), or of diatopic
origin, slightly different nominal forms for the same species as a result of
regionalisms (e.g., “cação-junteiro”, “juntão”, “junteiro” or
“tubarão-junteiro”).
Results
A total of 314 artisanal
fishers from 17 municipalities were interviewed (
18,47 ± 8,68 fishers/municipality), numbering a minimum of five fishers from
Primeira Cruz and a maximum of 35 fishers from Cândido Mendes. All fishers were
men, and most were from the state of Maranhão (90%; n = 282), mainly residing
in the municipalities of Cururupu, Cândido Mendes and Turiaçú, while other
fishers (10%; n = 32) were from other states, such as Ceará, Pará and Piauí.
Fishers’ age ranged from 20 to 83 years old (
47 years) and time acting as fishers ranged from two to 72 years ( 30 years).
All fishers
identify elasmobranchs as “leather fish,” informally classifying them in the
“sharks or cação family” or “ray family”. A total of 14 taxonomic families were
recorded (five shark and nine ray families), comprising 40 species (22 sharks
and 18 rays), resulting in 130 common names and an average of 3.25 names per
species (Figure 3; Table 1 and Table 2).
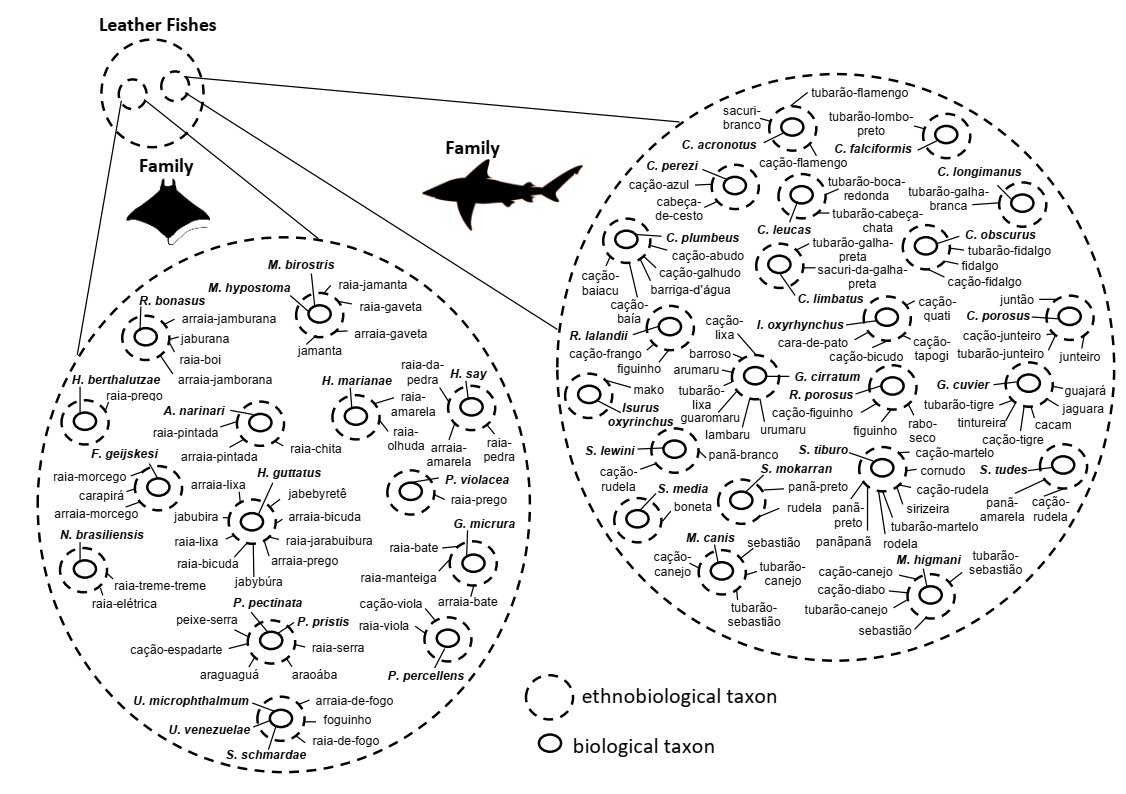
Figure 3 Hierarchical
diagram of shark and ray families with their common names associated to their
respective scientific names cited by artisanal fishers on the coast of the
state of Maranhão, located on the Brazilian Amazon Coast. Credit: Keyton K. F.
Coelho, 2021.
Regarding
linguistic considerations, homonyms occur most frequently among species
belonging to the same ray families (62%), such as Dasyatidae (Hypanus
berthalutzae and Pteroplatytrygon violacea), Mobulidae (Mobula
birostris and Mobula hypostoma), Pristidae (P. pristis and P.
pectinata) and Urotrygonidae (Urotrygon microphthalmum and Urotrygon
venezuelae) (Table 1). Homonyms between species from different families,
however, are also noted, such as Potamotrygonidae (Styracura schmardae)
and Urotrygonidae (U. microphthalmum and U. venezuelae) (Table
1). Regarding sharks, homonymy is most frequent (38%) for Carcharhinidae (Rhizoprionodon
lalandii and Rhizoprionodon porosus), Sphyrnidae (Sphyrna lewini,
Sphyrna mokarran, Sphyrna tiburo and Sphyrna tudes) and Triakidae (Mustelus
canis and Mustelus higmani) (Table 1).
Concerning
synonymy, averages of 3.18 and 3.33 names per species were identified for
sharks and rays, respectively (Table 4 and Table 5). Regarding sharks, Sphyrna
tiburo (Linnaeus, 1758) was given the greatest diversity of common names (n
= 8), displaying the highest relative frequency (11.43%) and citations (6.15%)
(Table 1, Table 2, and Table 4). Carcharhinus falciformis, C.
longimanus, Isurus oxyrinchus and Sphyrna media, on
the other hand, were all identified by a single common name throughout the
entire study area (Table 1 and Table 4). For rays, Hypanus guttatus
(Bloch & Schneider, 1801) was given the highest number of common names (n =
9), displaying the highest relative frequency (15%) and citations (6.92%)
(Table 1, Table 2 and Table 5). Interestingly, P. violacea and H.
berthalutzae were both recognized by the same common name (“raia-prego”)
(Table 1 and Table 5).
Table 4 List of shark
species with the number of synonyms, relative frequency (Fr%) and percentage of
citations by artisanal fishers from the Brazilian Amazon Coast.
|
Nº
|
Shark
|
Common
names
|
Fr%
|
% Citations
|
|
1
|
Sphyrna
tiburo
(Linnaeus, 1758)
|
8
|
11.43
|
6.15
|
|
2
|
Ginglymostoma
cirratum
(Bonnaterre, 1788)
|
7
|
10.00
|
5.38
|
|
3
|
Galeocerdo
cuvier
(Péron & Lesueur, 1822)
|
6
|
8.57
|
4.62
|
|
4
|
Carcharhinus
plumbeus (Nardo, 1827)
|
5
|
7.14
|
3.85
|
|
5
|
Mustelus
higmani
Springer & Lowe, 1963
|
5
|
7.14
|
3.85
|
|
6
|
Isogomphodon
oxyrhynchus (Müller & Henle, 1839)
|
4
|
5.71
|
3.08
|
|
7
|
Mustelus
canis (Mitchill,
1815)
|
4
|
5.71
|
3.08
|
|
8
|
Carcharhinus
porosus (Ranzani,
1839)
|
4
|
5.71
|
3.08
|
|
9
|
Carcharhinus
acronotus (Poey, 1860)
|
3
|
4.29
|
2.31
|
|
10
|
Carcharhinus
obscurus (Lesueur, 1818)
|
3
|
4.29
|
2.31
|
|
11
|
Rhizoprionodon
porosus (Poey,
1861)
|
3
|
4.29
|
2.31
|
|
12
|
Carcharhinus
leucas
(Müller & Henle, 1839)
|
2
|
2.86
|
1.54
|
|
13
|
Carcharhinus
perezi (Poey,
1876)
|
2
|
2.86
|
1.54
|
|
14
|
Rhizoprionodon
lalandii (Müller & Henle, 1839)
|
2
|
2.86
|
1.54
|
|
15
|
Sphyrna
lewini (Griffith & Smith, 1834)
|
2
|
2.86
|
1.54
|
|
16
|
Sphyrna
mokarran
(Rüppell, 1837)
|
2
|
2.86
|
1.54
|
|
17
|
Sphyrna
tudes
(Valenciennes, 1822)
|
2
|
2.86
|
1.54
|
|
18
|
Carcharhinus
limbatus (Müller & Henle, 1839)
|
2
|
2.86
|
1.54
|
|
19
|
Carcharhinus
falciformis (Müller & Henle, 1839)
|
1
|
1.43
|
0.77
|
|
20
|
Carcharhinus
longimanus (Poey, 1861)
|
1
|
1.43
|
0.77
|
|
21
|
Isurus
oxyrinchus Rafinesque, 1810
|
1
|
1.43
|
0.77
|
|
22
|
Sphyrna
media
Springer, 1940
|
1
|
1.43
|
0.77
|
|
TOTAL
|
70
|
100.00
|
|
Table 5 List
of ray species with the number of synonyms, relative frequency (Fr%) and
percentage of citations by artisanal fishers from the Brazilian Amazon Coast.
|
Nº
|
Ray
|
Common
names
|
Fr%
|
%
Citations
|
|
1
|
Hypanus
guttatus (Bloch & Schneider, 1801)
|
9
|
15.00
|
6.92
|
|
2
|
Pristis
pristis (Linnaeus, 1758)
|
5
|
8.33
|
3.85
|
|
3
|
Pristis
pectinata (Latham, 1794)
|
5
|
8.33
|
3.85
|
|
4
|
Mobula
birostris (Walbaum, 1792)
|
4
|
6.67
|
3.08
|
|
5
|
Mobula
hypostoma (Bancroft, 1831)
|
4
|
6.67
|
3.08
|
|
6
|
Rhinoptera
bonasus (Mitchill, 1815)
|
4
|
6.67
|
3.08
|
|
7
|
Fontitrygon
geijskesi (Boeseman, 1948)
|
3
|
5.00
|
2.31
|
|
8
|
Hypanus
say (Lesueur, 1817)
|
3
|
5.00
|
2.31
|
|
9
|
Gymnura micrura (Bloch & Schneider, 1801)
|
3
|
5.00
|
2.31
|
|
10
|
Urotrygon
microphthalmum Delsman, 1941
|
3
|
5.00
|
2.31
|
|
11
|
Urotrygon
venezuelae Schultz, 1949
|
3
|
5.00
|
2.31
|
|
12
|
Aetobatus
narinari (Euphrasen, 1790)
|
3
|
5.00
|
2.31
|
|
13
|
Styracura
schmardae (Werner 1904)
|
3
|
5.00
|
2.31
|
|
14
|
Hypanus marianae
(Gomes, Rosa & Gadig, 2000)
|
2
|
3.33
|
1.54
|
|
15
|
Narcine
brasiliensis (Olfers, 1831)
|
2
|
3.33
|
1.54
|
|
16
|
Pseudobatos
percellens (Walbaum, 1792)
|
2
|
3.33
|
1.54
|
|
17
|
Pteroplatytrygon
violacea (Bonaparte, 1832)
|
1
|
1.67
|
0.77
|
|
18
|
Hypanus
berthalutzae Petean, Naylor &
Lima 2020
|
1
|
1.67
|
0.77
|
|
TOTAL
|
60
|
100.00
|
|
Discussion
The richness of common names
(n = 130) in Portuguese or in Tupi-Guarani used by artisanal fishers does not
necessarily correspond to the number of biological shark or ray species, since
these common names are usually associated with polysemy, homonyms, or synonyms
cases when naming ethnospecies.
The polysemy
observed in the studied area is high and is generally applied when fishers
generically identify fish as “Cação”, “Panã”, “Raia” or “Arraia” or classify
them in the “shark” or “ray” family. These denominations do not correctly
define biological species but may reveal the biological diversity that exists
in the region. In the northeastern coast of Brazil, generic or polytypic taxa
are usually associated with the high species richness observed in some localities
(Barbosa-Filho et al. 2021; Previero et al. 2013) or categories of greater
economic or sociocultural importance in local fishing communities (Mourão and
Montenegro 2006; Pinto et al. 2015; Silvano and Begossi 2012). This was
verified in the present study, given the high richness of elasmobranch species
in the study area and the relevance of marine fish as a source of subsistence
and income on the coast of Maranhão.
Homonyms are more
frequent in rays (62%), mainly due to the phenotypic similarity usually
observed between different species belonging to the same family. An example of
this are the manta rays M. birostris and M. hypostoma, which are
locally identified as “arraia-gaveta”, “raia-gaveta”, “raia-jamanta” or simply
“jamanta”. Concerning sharks, although a lower homonym frequency is observed
(38%), a high morphological similarity between different species is also noted,
such as between M. canis and M. higmani known as “cação-canejo”,
“Sebastião”, “tubarão-canejo” or “tubarão-Sebastião”, as well as between R.
lalandii and R. porosus identified by “figuinho,” in addition to
“cação-frango” and “cação rabo-seco,” respectively. This same morphological
similarity pattern was observed by Carvalho et al. (2018) when studying the
ethnotaxonomy of sharks in the state of Rio Grande do Norte, Brazil, where R.
lalandii and R. porosus have also been recognized as “cação-frango”
and “cação rabo-seco”. This perception and recognition of biological groupings
by humans is based on similarities and differences shared between organisms,
but the skills required to recognize this variability must be developed (Barbosa-Filho
et al. 2021).
The synonymy
observed in sharks, with an average of 3.18 common names per species, was lower
than that observed by Barbosa-Filho et al. (2021) regarding the ethnotaxonomy
of sharks by fishers in the municipalities of Ilhéus, Una and Canavieiras, in
the state of Bahia, Brazil, which averaged 4.8 common names per species. These
authors indicated 13 common names for S. tiburo, higher than for the
same species in our study (n = 8). These differences in common names are often
justified by geographic variations, linguistic differences, or person to person
changes (Carvalho et al. 2018; Freire and Carvalho-Filho 2009; Freire and Pauly
2005; Last et al. 2016). However, when analyzing the popular knowledge of
artisanal fishers concerning 22 shark species, Carvalho et al. (2018) also identified
an average of 3.17 common names per species. For stingrays, the highest number
of common names for H. guttatus (n = 9; five in Portuguese and four in
Tupi-Guarani) may be associated with the use of ethnotaxonomic characteristics
in their identification (e.g., stingray) and their high occurrence along the
study area (as reported by some fishers: “... just cast the net from one end of
the coast to the other and you catch this fish”), favoring its availability and
commercial value accessible to local consumers.
The national
average for Brazil is of six common names for each biological species, but some
fish species are known by more than 30 common names, in addition to
higher-level taxonomic groups that include different families, genera, and
species that are referred to by a single common name (Freire and Pauly 2005),
as in the case of rays (“raia” or “arraia” in Brazilian Portuguese). For taxa
displaying high synonymy, the insertion of “notes” is recommended for reviews,
catalogs, and other publications, to avoid naming errors (Papavero 1994).
Many common fish
names reflect fisher local ecological knowledge (Mourão and Barbosa-Filho
2018). All artisanal fishers who participated in this study have fishing as
their main activity and demonstrate knowledge concerning the biology of the
fish they often catch. This is reflected in the length of experience in the
fishing profession ( 30 years), where the use of
natural aquatic resources is the result of life experience and knowledge. These
social actors have empirical knowledge that must be respected regarding their
behavior in relation to the environment when obtaining resources (Mourão and
Nordi 2002) with a wealth of information on the biology, ecology and etymology
of different groups of animals (Mourão and Barbosa-Filho 2018; Silvano and
Begossi 2012). This knowledge is paramount regarding the relational composition
of social existence, being transmitted orally and through experience to
descendants over time in the construction of identity bonds across generations
(Aragão 2021; Aragão et al. 2019).
The association
of ethnotaxonomic characteristics favors the existence of many common names in
Portuguese (87%) for shark and ray identification. Morphological aspects are
the most considered for naming species, highlighting the size or shape of the
body, or the texture and colors of body parts, which are usually associated
with a word (noun or adjective) to designate the species. An apt example is the
“cação-bicudo” or “cara-de-pato” (transliteration Portuguese to English =
“beaked shark” or “duckface shark”, respectively). Daggernose shark I.
oxyrhynchus, which, according to fishers, is named after the shape of its
head: “This shark has a head that thins and flattens up to the beak”. The
Sharpsnout stingray F. geijskesi receives the composite name of
“raia-morcego” (“bat stingray”) due to the presence and span of its large fins.
Barbosa and Nascimento (2008) suggest that the use of common names related to
other animals, objects or actions should be composed to avoid confusion and
thus, aid in informal species identification. Thus, the use of nouns and
adjectives when establishing compound or derivative names is extremely
important for the determination of a specific taxon (Papavero 1994).
In some cases,
different morphological characteristics are considered in the complete naming
of the species, as in the case of the “panã-amarelo”/Smalleyer hammerhead
(“yellow panã”) S. tudes, in which fishers relate the shape of the head
with the characteristic color of the animal: “This cação has a hammer-shaped
head and is yellow on the underside of the head and the rest of the body”. In
other situations, body shape can confuse fishers as to the difference between
some species of rays and sharks, as verified in the statement that the
“raia-viola”/Chola guitarfish P. percellens and the
“raias-serras”/Sawfishes P. pristis or P. pectinata are usually
identified as “cação-viola” and “cações-espadartes”, respectively, attributing
these names due to their similarity with cações (sharks).
The color pattern
is the second most applied morphological aspect in species identification, such
as in the “sacuri-branco”/Blacknose shark C. acronotus (“with a black
marking on the tip of the nose”) and the Tiger shark G. cuvier (“with
markings along the body”), or the “raia-manteiga”/Smooth butterfly ray G.
micrura (“yellowish color on the underside of the body”), the
“raia-pintada”/Whitespotted eagle ray A. narinari (“all the upper part
of this species has white spots”), and the “manta ray”/Giant ray M.
birostris (“with some white spots near the head”). Colors play a major role
in descriptions and are important for the identification of the vast majority
of plant or animal organisms (Papavero 1994). In fact, this physical feature
stands out to the eye, being frequently used in the construction of popular and
vernacular denominations (Martins 2015; Mourão and Barbosa-Filho 2018).
The size and
texture of the body are morphological aspects evidenced in species such as the
“sacuri-branco”/Blacknose shark C. acronotus (“...it is small, when
large it reaches one meter...”) and in the Longnose stingray H. guttatus
(“its leather is sandpaper..., you can even scrape the hull of the boat”).
Names in Tupi-Guarani also reveal the same aspects, such as “jaguara,” “cacam”
or “guajará” (“large fish, of enormous size,” referring to the Tiger shark G.
cuvier) or “jabubira,” “jabebyretê,” “jabybúra” or “ray-jarabuibura”
(“swelled, lumpy or blistered skin,” referring to the stingray H. guttatus).
However, these and other names in the Tupi-Guarani language used to identify
sharks and rays, such as “arumaru,” “guaromaru,” “lambaru” or “urumaru” (G.
cirratum), “panãpanã” or “panã” (S. tiburo) and “araguaguá” or
“araoába” (P. pristis and P. pectinata) are no longer used by fishers in
the region. These names are generally used by fishers aged between 50 and 80
years due to contact with older fisher generations (e.g., parents and
grandparents). A loss of cultural values through applied names is
verified, due to the lack of interest of young people in fishing. For Pinto et
al. (2015), this lack of interest occurs due to the lack of investment in
storing, processing, and marketing fish, in addition to low values and the
search for new employment opportunities.
Morphological
characteristics were also widely applied in early descriptions of the local
aquatic fauna in colonial periods, as observed for the “tubarão-lixa”/Nurse
shark G. cirratum ("...the hide of this dogfish is like
sandpaper" or "...obtuse snout, somewhat small, the same is noted for
the eyes, located in the upper third of the head...”), the hammerhead
shark/Bonnethead S. tiburo (“... semicircular cephalic contour and the
nostrils are close to the eyes...”), the spotted ray/Whitespotted eagle ray A.
narinari (“...this fish is all spotted white and black”) and the
“raia-bicuda”/Longnose stingray (beaked ray) H. guttatus (“...the adults
have a series of spines on the midline of the body, to the tail dart...").
The ecological
criteria used by fishers reveal much of the habitat of some species, such as
Nurse shark G. cirratum (“it likes muddy environments”), the
“panã-branco”/Scalloped hammerhead S. lewini (“it is found out there, in
high seas”) and the “raia-pedra”/Bluntnose stingray (“rock ray”) H. say
(“its likes stony bottoms”). The behavioral and physiological criteria reported
by fishers indicate certain peculiar characteristics of some species, as
observed for the “tubarão-boca-redonda”/Bull shark (“roundmouth shark”) C.
leucas, which emits sounds, making a lot of noise under the boat and is
highly resistant when caught, even tearing nets or breaking longlines, the
“electric ray”/Brazilian electric ray N. brasiliensis, capable of
producing painful electrical discharges that leave fisher body parts numb for
long periods of time, and the “raias-de-fogo” (“fire rays”) Chupare stingray S.
schmardae and Smalleyed round stingray U. microphthalmum that can
leave irreparable injuries when piercing the human legs, arms or hands with
their stingers (see Carvalho et al. 2019; Dias et al. 2016 and Junior et al.
2013).
These
ethnotaxonomic fish identification patterns are also reported in other
ethnobiological studies (Mourão and Barbosa-Filho 2018; Mourão and Nordi 2002,
2003; Pinto et al. 2016), but morphological criteria are generally the most
employed in elasmobranch identification and naming (Barbosa-Filho et al. 2021;
Carvalho et al. 2018; Pinto et al. 2016).
All the
ethnospecies mentioned by the interviewed fishers match those mentioned in the
preexisting literature (Almeida 2006; Almeida 2008; Araujo and Gonçalves 2006;
Almeida et al. 2011; Barbosa 1951; Carvalho 1964; D’Abbeville 2008; Fortes and
Galvão 2006; ICMBIO 2018; Marceniuk et al. 2020; Martins-Jura et al. 1987;
Nunes and Santos 2006; Nunes et al. 2005; Nunes et al. 2011; Papavero et al.
2000; Silva and Paz 2006; Spix and Martius 1829; Stride et al. 1992), with the
exception of the “raia-morcego”/Sharpsnout stingray F. geijskesi, which
was also identified by the name “Carapirá” in the municipality of Carutapera.
However, some fisher reports (65%; n = 205) indicate that they had never caught
or seen a P. pristis or P. pectinata specimen throughout their
years of fishing experience (e.g., “I only hear about this animal, but I've
never seen it, I’d like to see it...”). The few reports (34.72%) concerning species of Pristidae function as historical
records of the distribution of their populations, indicating occurrence and
capture sites of these animals, since the information is brought by the oldest
fishers in the region and indicate a long time since the last time these
animals were seen (“...but it has been a long time since they appears in these
waters”; “About three years ago one appeared here, half a meter in size...”).
Fishers from Batoque Beach in the state of Ceará,
northeastern Brazil, reported that the sawfish P. pristis has not
been observed in the region for over 40 years (Pinto et al. 2015). In general,
fisher reports indicate how much these species have been suffering population
declines over the years. Feitosa et al. (2017) recorded 23 sawfish catches in the region Maranhão Amazon
coast between 1984 and 2016 and demonstrated that the degradation of these
species’ habitat through mangrove deforestation, pollution and strong artisanal
fishing pressures are the main factors responsible for the observed declines.
The
high diversity of common names used in Brazil to designate fish species is a
challenge for adequate collection of fish landing data (Freire and Pauly 2003).
In this sense, the designation of a certain species by several popular names,
as well as the use of the same epithet to refer to different species, makes it
difficult to record species-specific fish in existing landing monitoring
systems, a fact that limits the possibilities for assessing the impact of
fisheries on fishery resource populations (Freire and Pauly 2005). For example,
the ethnocategory “cação” is used to designate a multitude of scientific
species from different shark families, and this category is usually used in regional
fisheries monitoring systems in Brazil to group all locally caught shark
species (Freire and Pauly 2005; Barbosa-Filho et al. 2021). Such a procedure is
not very useful in terms of fisheries management, as it makes a basic
assessment of the population dynamics of the different fishing resources
exploited over time unfeasible (Freire and Pauly, 2003), a fact that strongly
restricts the possibilities of the Brazilian State to adequately manage the
fishing for elasmobranchs.
It is verified
that, in Brazil, it is usual to group the fishing landings of elasmobranchs
under the generic categories “cações” and “arraias” in several official
documents such as evaluations of landings carried out by the public
authorities, as well as in research reports and scientific articles
(Barbosa-Filho et al. 2021; Medeiros et al. 2022). It is possible that the
challenges inherent in the taxonomic identification of elasmobranch species,
the fact that sharks are normally landed eviscerated and headless, and the
possible negligence of researchers and fisheries managers in carrying out a
thorough job of identifying the landed elasmobranch species, culminate for this
scenario. Given this context, for a more adequate management of the
elasmobranch fishery in the country, it is essential to link academic knowledge
from scientists and fisheries managers with those related to the ethnotaxonomy
developed by fishermen for the construction of a landing data collection system
more judicious and fruitful, that is, that seek to carry out the
species-specific identification of the captured animals.
Conclusion
The diversity of common names
used to identify different shark and ray species from the Brazilian Amazon
Coast is a consequence of the high miscegenation rates that took place between Indigenous
and settler populations during the colonization process. This linguistic
richness is easily observed by homonyms and synonyms that reflect a series of
ethnotaxonomic characteristics employed for species identification. The use of
these common names facilitates traditional fishing community communication with
consumers and civil society. On the other hand, this is one of the main
difficulties regarding correct species identification. Thus, constant updates
concerning common names should take place, in order to standardize species
nomenclature in the region. Finally, fisher knowledge regarding shark and ray
names can contribute to basic information on elasmobranchs captured throughout
the coast of Maranhão and species-specific recognition in fishing landing
monitoring systems, generating subsidies for the development of conservation
and management plans for these fishery resources.
Author contributions section
KKFC and JLSN, conceived and
planned the study; KKFC, GR, MLVBF, NW, AROPN and JLSN reviewed and analyzed
the data; KKFC, GR, AMB, MLVBF, NW, RMSB, AROPN and JLSN wrote the paper.
Acknowledgements
To the artisanal fishermen for
their willingness to participate in this study, to Marcelo Neves Diniz for the
search for historical documents, to Brenda Soares da Silva Nunes for making the
map, for the financial support to JLSN through the Fundação de Amparo à
Pesquisa do Maranhão (FAPEMA - BEPP- 02106/18; BPD-04215/17;
AQUIPESCA-06605/16), Biodiversity Conservation: interface between the creative
economy and environmental quality (CAPES - Aid Nº 0762/2020, Process Nº 88881.510069/2020-01),
and the Coordination for the Improvement of Higher Education Personnel (CAPES)
and the Graduate Program in Biodiversity and Biotechnology in the Legal Amazon
- BIONORTE Network.
Declarations
Permissions: This research followed the
guidelines set by the Declaration of Helsinki and Tokyo for humans and was
approved by the Human Ethics Committee of the Federal University of Maranhão
(UFMA - nº 3717163 - CAAE 25628919.9.0000.5087), Brazilian Institute for the
Environment and Renewable Natural Resources (IBAMA; SISBIO - nº 60306-1) and
the State Secretariat for the Environment and Natural Resources
(Superintendence of Biodiversity and Protected Areas; SEMA-MA - nº 00397/2019).
All interviewees signed a Free and Informed Consent Term (FICT).
Sources of funding: None declared.
Conflicts of Interest: None declared.
References Cited
Almeida, Z. S.
2008. Os recursos pesqueiros marinhos e estuarinos do Maranhão: Biologia,
Tecnologia, Socioeconomia, Estado da Arte e Manejo. Tese (Doutorado), Curso em
Zoologia, Universidade Federal do Pará/ Museo Paraense Emílio Goeldi.
Almeida, Z. S. 2006. Um dia do peixe,
outro do pescador. In Elasmobrânquios da costa maranhense: história
evolutiva, biologia e pesca, edited by Z. S. Almeida and R. Fortes, pp.
60–69. São Luís, UEMA.
Almeida, Z. S., Frédou, F. L., Nunes,
J. L. S., Lessa, R. P., and Pinheiro, A. L. R. 2011. Biodiversidade de
Elasmobrânquios. In Peixes marinhos e estuarinos do Maranhão, edited by
Nunes, J. L. S. and Piorski, N. M. pp. 37–94. Editora Café & Lápis, São
Luís.
Almeida, Z. S., Nunes, J. S., and
Costa, C. L. 2016. Presencia De Urotrygon Microphthalmum (Elasmobranchii:
Urolophidae) En Aguas Bajas De Maranhão (Brasil) Y Notas Sobre Su Biologia. Boletín
de Investigaciones Marinas y Costeras 29:67–72.
DOI:10.25268/bimc.invemar.2000.29.0.314
Amorim, M. A. 2005. Os
Franciscanos no Maranhão e Grão-Pará: missão e cultura na primeira metade de
seiscentos. Centro de estudos de história religiosa, Universidade Católica
Portuguesa, Lisboa, 362p.
Aragão, M. C. O. 2021. Cultura
e conhecimento tradicional faces possíveis da sustentabilidade. In Unidades
de conservação e comunidades tradicionais: Desafios da sobrevivência dos
espaços e identidades, edited by Souza, R. M., Santos, S. S. C., Santos, E.
A., and Aragão, M. C. O. pp. 27–42. 1ª ed. Criação Editora, Aracaju, Sergipe.
Aragão, G. M. O., Oliveira, G. P.,
Kotas, J. E., and Spach, H. L. 2019. O Conhecimento Ecológico Local Dos
Pescadores Artesanais Sobre Os Elasmobrânquios Marinho-Costeiros Na Apa Do
Delta Do Parnaíba, Nordeste Do Brasil. Arquivos de Ciências do Mar
52:34–49. DOI:10.32360/acmar.v52i1.33667
Araujo, C. E., and Gonçalves, F. S.
2006. Como os elasmobrânquios se reproduzem. In Elasmobrânquios da costa
maranhense: história evolutiva, biologia e pesca, edited by Z. S. Almeida
and R. Fortes, pp. 28–36. São Luís, UEMA.
Araujo, N. L. F., Lopes, C. A.,
Brito, V. B., Santos, L. N., Barbosa-Filho, M. L. V., Amaral, C. R. L.,
Siciliano, S., and Hauser-Davis, R. A. 2020. Artisanally Landed
Elasmobranchs Along the Coast of Rio De Janeiro, Brazil. Boletim
do Laboratório de Hidrobiologia 30:33–53.
DOI:10.18764/1981-6421e2020.4
Barbosa, A. L. 1951. Pequeno
vocabulário tupi-português. Livraria São José, Rio de Janeiro.
Barbosa-Filho, M. L. V.,
Hauser-Davis, R. A., Siciliano, S., Dias, T. L. P., Alves, R. R. N., and
Costa-Neto, E. M. 2019. Historical Shark Meat Consumption and Trade Trends
in a Global Richness Hotspot. Ethnobiology Letters 10:97–103. DOI:10.14237/ebl.10.1.2019.1560
Barbosa-Filho, M. L. V., Ramires, M.,
Mourão, J. S., Rosa, R. S., Alves, R. R. N., and Costa-Neto, E. M. 2021. Ethnotaxonomy
of Sharks by Expert Fishers from South Bahia, Brazil: Implications for Fisheries
Management and Conservation. Ethnobiology and Conservation 10:1–12.
DOI:10.15451/ec2021-08-10.02-1-12
Barbosa, J. M., and Nascimento, C. M. 2008. Sistematização de nomes vulgares de peixes comerciais do Brasil: 2
espécies marinhas. Revista Brasileira de Engenharia de Pesca, São Luís,
v. 3, n. 3, pp. 76–90. Available at:
https://ppg.revistas.uema.br/index.php/REPESCA/article/view/100/100. Accessed on May 24, 2021.
Carvalho, J. P. 1964. Comentários
sobre os peixes mencionados na obra “história dos animais e árvores do
Maranhão” de frei Cristovão de Lisboa. Arq. Est. Biol. Mar. Fortaleza,
4:1–39.
Carvalho, I. E. M., Costa, J. A.,
Júnior, V. H., Silva, G. V. F., and Nunes, J. L. S. 2019. Acidentes causados
por raias em pescadores artesanais no Estado do Maranhão. In Tópicos
integrados de zoologia, edited by Júnior, J. M. B. O. and Calvão, L. B. pp.
26–35. Ponta Grossa, Atena Editora, Paraná.
Carvalho, M. M., Oliveira, M. R.,
Lopes, P. F. M., and Oliveira, J. E. L. 2018. Ethnotaxonomy of Sharks
from Tropical Waters of Brazil. Journal of Ethnobiology and Ethnomedicine 14:1–12.
DOI:10.1186/s13002-018-0273-0
Castro, A. C. L., Azevedo, J. W. J.,
Ferreira, H. R. S., Soares, L. S., Pinheiro-Júnior, J. R., Smith, L. M. R.,
Silva, M. H. L. 2019. Feeding Activity of the Cayenne Pompano Trachinotus
cayennensis (Cuvier 1832) (Perciformes, Carangidae) in Estuaries on the Western
Coast of the State of Maranhão, Brazil. Brazilian
Journal of Biology 79(2):311–320.
DOI:10.1590/1519-6984.182683
D’Abbeville,
C. 2008. História da missão dos padres Capuchinhos na ilha do Maranhão e terras
circunvizinhas. Tradução de Sérgio Milliet. Brasília, DF: Senado Federal,
Conselho Editorial, 105, 404p. Available at:
https://www2.senado.leg.br/bdsf/bitstream/handle/id/576068/000838911_Historia_padres_capuchinhos_Maranhao.pdf?sequence=1&isAllowed=y.
Accessed February 24, 2021.
Dias, H. N., Avelar, R. F., and
Santos, R. V. E. 2016. Etnoictiologia de arraias nas comunidades pesqueiras de
Porto grande e Vila de Cuiarana, Salinópolis, Pará - Brasil. Engrenagem,
Belém, ano VI, n. 12, pp. 52–71. ISSN 2236-4757.
Dietrich, W. 2016. O Tronco tupi e as
suas famílias de línguas. In O português e o tupi no Brasil, edited by
Dietrich, W. and Noll, V. pp. 9–25. Editora Contexto, São Paulo.
Dietrich, W., and Noll, V. 2016a. O
papel do tupi na formação do português brasileiro. In O português e o tupi
no Brasil, edited by Dietrich, W. and Noll, V. pp. 81–104. Editora
Contexto, São Paulo.
Dietrich, W., and Noll, V. 2016b. Prefácio.
In O português e o tupi no Brasil, edited by Dietrich, W. and Noll, V.
pp. 7–8. Editora Contexto, São Paulo.
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh,
R. D., Kyne, P. M., Harrison, L. R., Carlson, J. K., Davidson, L. N., Fordham,
S. V., Francis, M. P., Pollock, C. M., Simpfendorfer, C. A., Burgess, G. H.,
Carpenter, K. E., Compagno, L. J., Ebert, D. A., Gibson, C., Heupel, M. R.,
Livingstone, S. R., Sanciangco, J. C., Stevens, J. D., Valenti, S., and White,
W. T. 2014. Extinction Risk and Conservation of the World’s Sharks and Rays. Elife
3:1–34. DOI:10.7554/elife.00590
Dulvy, N. K., Pacoureau, N., Rigby, C. L., Pollom, R.
A., Jabado, R. W., Ebert, D. A., Finucci, B., Pollock, C. M., Cheok, J.,
Derrick, D. H., Herman, K. B., Sherman, C. S., VanderWright, W. J., Lawson, J.
M., Walls, R. H. L., Carlson, J. K., Charvet, P., Bineesh, K. K., Fernando, D.,
Ralph, G. M., Matsushiba, J. H., Hilton-Taylor, C., Fordham, S. V., and
Simpfendorfer, C. A. 2021. Overfishing Drives over One-Third of All Sharks and Rays
Toward a Global Extinction Crisis. Current Biology 31(21):1–15. DOI:10.1016/j.cub.2021.08.062
El-Robrini, M., Santos, J. H. S.,
Lima, L. G., Santos, A. L. S., Santos, M. C. F. V., and Souza, U. D. V., 2018.
Maranhão. In Panorama da erosão costeira no Brasil, edited by Dieter, M.
pp. 167–239. Brasília, DF: MMA.
Feitosa, L. M., Martins, A. P. B.,
and Nunes, J. L. S. 2017. Sawfish (Pristidae) Records Along the Eastern
Amazon Coast. Endangered Species Research 34:229–234.
DOI:10.3354/esr00852
Ferreira-Araujo, T., Lopes, P. F. M., and Lima, S. M.
Q. 2021. Size matters: Identity of Culturally Important Herrings in Northeastern
Brazil. Ethnobiology and Conservation 10:1–30.
DOI:10.15451/ec2020-11-10.07-1-29
Fortes R. and Galvão, M. 2006. Ataque
ou instinto de sobrevivência. In Elasmobrânquios da costa maranhense:
história evolutiva, biologia e pesca, edited by Z. S. Almeida and R.
Fortes, pp. 46–59. São Luís, UEMA.
Freire, K. M. F., and Carvalho-Filho,
A. 2009. Richness of Common Names of Brazilian Reef Fishes. Pan-American
Journal of Aquatic Sciences 4:96–145.
Freire, K. M. F., and Pauly, D. 2005. Richness of Common
Names of Brazilian Marine Fishes and its Effect on Catch Statistics. Journal
of Ethnobiology 25:279–296.
DOI:10.2993/0278-0771(2005)25[279:ROCNOB]2.0.CO;2
Giareta, E. P., Prado, A. C., Leite, R. D., Padilha,
É., Santos, I. H., Wosiak, C. D. C. D. L., and Wosnick, N. 2021. Fishermen’s Participation
in Research and Conservation of Coastal Elasmobranchs. Ocean and Coastal
Management 199:1–9. DOI:10.1016/j.ocecoaman.2020.105421
Gonçalves, F. S. 2004. Pesca,
reprodução e alimentação de Rhizoprionodon porosus Poey, 1861
(Elasmobranchii; Carcharhinidae) na Plataforma Continental Maranhense.
Monografia (Graduação), Universidade Estadual do Maranhão, São Luís.
IBGE. 2020. Instituto Brasileiro de
Geografia e Estatística. Cidades e Estados. Available at:
https://cidades.ibge.gov.br/brasil/ma/panorama. Accessed March 18, 2021.
ICMBio. 2018. Livro Vermelho da Fauna
Brasileira Ameaçada de Extinção: Volume VI–Peixes. In Livro Vermelho da
Fauna Brasileira Ameaçada de Extinção, edited by Instituto Chico Mendes de
Conservação da Biodiversidade. Ministério do Meio Ambiente, Brasília.
IMESC, 2020. Unidades de
Conservação Estaduais. Instituto Maranhense de Estudos Socioeconômicos e
Cartográficos. São Luís: IMESC, 70p.
Junior, V., Cardoso, J. L. C., and
Neto, D. G. 2013. Injuries by Carine and Freshwater Stingrays: History, Clinical
Aspects of the Envenomations and Current Status of a Neglected Problem in
Brazil. Journal of Venomous Animals and Toxins including Tropical Diseases
19:1–16. DOI:10.1186/1678-9199-19-16
Ladislau, D. S., Souza, P. L., Aride,
P. H. R., Oliveira, A. T., and Gubiani, É. A. 2021. Current situation
and future perspectives of ethnoichthyology in Brazil. Ethnobiology and
Conservation 10:09. DOI:10.15451/EC2020-11-10.09-1-35
Last, P. R., White, W. T., Carvalho, M. R., Séret, B.,
Stehmann, M. F. W., and Naylor, G. J. P. 2016. Rays of the World.
Cornell University Press.
Lessa, R. P., Santana, F., Menni, R., and Almeida, Z.
1999. Population Structure and Reproductive Biology of the Smalltail Shark (Carcharhinus
porosus) off Maranhão (Brazil). Marine and Freshwater Research 50:383–388.
DOI:10.1071/MF98127
Lessa, R. P., and Silva, T. C. 1992. Fecundity and Reproductive
Cycle of the Bonnethead Shark Sphyrna tiburo (Linnaeus, 1758) from
Northern Brazil. Revista Brasileira de Biologia 52(4):533–545.
Marceniuk, A. P., Barthem, R. B.,
Wosiacki, W. B., Klautau, A. G. C. M., Junior, T. V., Rotundo, M. M., Cordeiro,
A. P. B., Romão-Júnior, J. G., Santos, W. C. R., Reis, T. S., Muniz, M. R.,
Cardoso, G. S., and Viana, S. T. F. L. 2020. Sharks and Batoids
(Subclass Elasmobranchii) Caught in the Industrial Fisheries off the Brazilian
North Coast. Revista Nordestina de Biologia 27:120–142. DOI:10.22478/ufpb.2236-1480.2019v27n1.47112
Martins, S. C. 2015. A sinonímia,
a polissemia e a homonímia no vocabulário da Fauna e da Flora. Estudos
Linguísticos, São Paulo, 44(1):186–207.
Martins, A. P. B., Feitosa, L. M.,
Lessa, R. P., Almeida, Z. S., Heupel, M., Silva, W. M., Tchaicka, L., and
Nunes, J. L. S. 2018. Analysis of the Supply Chain and Conservation Status
of Sharks (Elasmobranchii: Superorder Selachimorpha) Based on Fisher Knowledge.
PLoS One 13:1–15.
DOI:10.1371/journal.pone.0193969
Martins-Juras, I. A. G., Juras, A.
A., and Menezes, N. A. 1987. Relação preliminar dos peixes da Ilha de São Luís,
Maranhão, Brasil. Revista Brasileira de Zoologia 4:105–113. DOI:10.1590/s0101-81751987000200003
Medeiros, M. C., Pinto, A. S.,
Santos, D. R., Martel, G., Lopes, S. F., and Mourão, J. S. 2022. Folk Taxonomy
and Scientific Nomenclature: Working Together for Conservation of Fishery Resources
in Brazil. Journal for Nature Conservation 68:126214.
DOI:10.1016/j.jnc.2022.126214
Minelli, A., 1999. The Names of Animals. Trends
Ecology Evolution 14(12):462–463. DOI:10.1016/s0169-5347(99)01747-4
Mourão, J. S., and Barbosa-Filho, M.
L. V. 2018. Ethnotaxomy as a Methodological Tool for Studies of the
Ichthyofauna and its Conservation Implications: A Review. Ethnozoology
Animals in our Lives 71–94. DOI:10.1016/B978-0-12-809913-1.00006-5
Mourão, J. S., and Montenegro, S. C. S. 2006. Pescadores e Peixes: O conhecimento local e o uso da taxonomia folk
baseado no modelo berliniano. 1 ed. Série Estudos e
Debates, Recife, PE, Brazil.
Mourão, J. S., and Nordi, N. 2003.
Etnoictiologia De Pescadores Artesanais Do Estuário Do Rio Mamanguape, Paraíba,
Brasil. Boletim do Instituto de Pesca. São Paulo, 29(1):9–17.
Mourão, J. S., and Nordi, N. 2002.
Principais critérios utilizados por pescadores artesanais na taxonomia folk dos
peixes do estuário do rio mamanguape, Paraíba-Brasil. Interciencia 27:607–612.
Nunes, J. L. S., Almeida, Z. S., and
Piorski, N. M. 2005. Raias capturadas pela pesca artesanal em águas rasas do
Maranhão - Brasil. Arquivos de Ciências do Mar 38:49–54.
Nunes, J. L. S., Rincon, G., Piorski,
N. M., and Martins, A. P. B. 2016. Near-term Embryos in a Pristis
pristis (Elasmobranchii: Pristidae) from Brazil. Journal of Fish Biology
89:1112–1120. DOI:10.1111/jfb.12946
Nunes, J. L. S., and Santos, N. B.
2006. Dos tubarossauros aos modernos tubarões: história evolutiva. In Elasmobrânquios
da costa maranhense: história evolutiva, biologia e pesca, edited by Z. S.
Almeida and R. Fortes, pp. 11–27. São Luís, UEMA.
Nunes, J. L. S., Silva, S. K. L., and
Piorski, N. M. 2011. Lista de peixes marinhos e estuarinos do Maranhão. In Peixes
marinhos e estuarinos do Maranhão, edited by Nunes, J. L. S. and Piorski,
N. M. pp. 181–201. Editora Café & Lápis, São Luís.
Pacoureau, N., Rigby, C. L., Kyne, P.
M., Sherley, R. B., Winker, H., Carlson, J. K., Fordham, S. V.,
Barreto, R., Fernando, D., Francis, M. P., Jabado, R. W., Herman, K. B., Liu,
K. M., Marshall, A. D., Pollom, R. A., Romanov, E. V., Simpfendorfer, C. A.,
Yin, J. S., Kindsvater, H. K., and Dulvy, N. K. 2021. Half a Century of Global Decline
in Oceanic Sharks and Rays. Nature 589:567–571. DOI:10.1038/s41586-020-03173-9
Papavero, N. 1994. Fundamentos
práticos de taxonomia zoológica: coleções, bibliografia, nomenclatura. 2
ed. São Paulo: Editora da Universidade Estadual Paulista. 285p.
Papavero, N., Teixeira, D. M.,
Overal, W. L., and Pujol-Luz, J. R. 2000. O Novo Éden: a fauna da Amazônia
brasileira nos relatos de viajantes e cronistas desde a descoberta do rio
Amazonas por Pinzón (1500) até o Tratado de Santo Idelfonso (1777). Belém:
Museu Paraense Emílio Goeldi, 381p.
Pinto, M. F., Mourão, J. S., and
Alves, R. R. N. 2015. Use of Ichthyofauna by Artisanal Fishermen at Two Protected
Areas Along the Coast of Northeast Brazil. Journal of Ethnobiology and
Ethnomedicine 11(20):1–32. DOI:10.1186/s13002-015-0007-5
Pinto, M. F., Mourão, J. S., and Alves, R. R. N. 2016.
How do Artisanal Fishermen Name Fish? An Ethnotaxonomic Study in Northeastern
Brazil. Journal of Ethnobiology 36:348–381.
DOI:10.2993/0278-0771-36.2.348
Prazeres, Frei Francisco de Nossa
Senhora dos, 1891. Poranduba maranhense, ou relação histórica da província
do Maranhão [...] com [...] um dicionário abreviado da língua geral do Brazil.
Revista Trimensal do Instituto Histórico e Geographico Brazileiro. v. 54, pt.
1, p. [4]–277. [Inclui ‘Nota sobre o Poranduba Maranhense’, de César Augusto
Marques, p. 279-281.]. Available at:
http://etnolinguistica.wdfiles.com/local--files/biblio%3Aprazeres-1891poranduba/prazeres
1891 _poranduba.pdf. Accessed on February 25, 2021.
Previero, M., Minte-Vera, C. V., and
Moura, R. L. 2013. Fisheries Monitoring in Babel: Fish Ethnotaxonomy in
a Hotspot of Common Names. Neotropical Ichthyology 11:467–476. DOI:10.1590/S1679-62252013000200016
Rodrigues, A. D. I. 2016. Tupi,
tupinambá, línguas gerais e o português no Brasil. In O português e o tupi
no Brasil, edited by Dietrich, W. and Noll, V. pp. 27–48. Editora Contexto,
São Paulo.
Rodrigues, C. A. L., Carvalho, I. F.
S., Costa, J. F., Queirós, K. B. N., Nunes, L. R., and Almeida, Z. S. 2021.
Etnoconhecimento dos pescadores artesanais de Santo Amaro - Maranhão: aspectos
relacionados à pesca e biologia da ictiofauna de valor comercial na região. Revista
Arquivos Científicos (IMMES) 4:97–106.
Silva, Á. P. C., Costa, N. M. S.,
Silva, M. C. S., Santos, R. P., Gomes, I. O., Gomes, J. B., and Almeida, Z. S.
2021. Etnoconhecimento de pescadores artesanais na comunidade Bebedouro, Santo
Amaro, Brasil. Research, Society and Development 10(8):1–9,
e52510817545. DOI:10.33448/rsd-v10i8.17545
Silva, C. M. L., and Paz, A. C. 2006. O comportamento alimentar dos elasmobrânquios. In Elasmobrânquios
da costa maranhense: história evolutiva, biologia e pesca, edited by Z. S.
Almeida and R. Fortes, pp. 37–45. São Luís, UEMA.
Silvano, R. A. M., and Begossi, A.,
2012. Fishermen’s Local Ecological Knowledge on Southeastern Brazilian Coastal
fishes: Contributions to Research, Conservation, and Management. Neotropical
Ichthyology 10:133–147. DOI:10.1590/S1679-62252012000100013
Spix, J. B., and Martius, K. F. P., 1829. Selecta
genera et species piscium quos in itinere per brasiliam annis mdcccxvii –
mdcccxx. Available at:
https://digital.bbm.usp.br/view/?45000009046&bbm/4274#page/1/mode/2up.
Accessed on February 24, 2021.
Stride, R. K., Batista, V. S., and Raposo, L. A. 1992.
Pesca experimental de tubarão com redes de emalhar no
litoral maranhense. São Luís: CORSUP/EDUFMA.
Viana, J. S., and Souza, R. F. C.
2019. A Pesca Artesanal Com Espinhel De Fundo Na Plataforma Continental
Amazônica. Arquivos de Ciências do Mar 52:21–33.
DOI:10.32360/acmar.v52i1.33408
Wosnick, N., Nunes, A. R. O. P.,
Feitosa, L. M., Coelho, K. K. F., Brito, R. M. S., Martins, A. P. B., Rincon,
G., and Nunes, J. L. S. 2019. Revisão sobre a diversidade, ameaças e
conservação dos elasmobrânquios do Maranhão. In Tópicos integrados de
zoologia, edited by Júnior, J. M. B. O. and Calvão, L. B. pp. 44–54. Ponta
Grossa, Atena Editora, Paraná.
ACCESS